750,000+
AtriClip Devices Sold
A Simplified Approach to Left Atrial Appendage Exclusion

The AtriClip Left Atrial Appendage Exclusion (LAAE) System is indicated for the exclusion of the heart’s Left Atrial Appendage (LAA), performed under direct visualization and in conjunction with other cardiac surgical procedures.
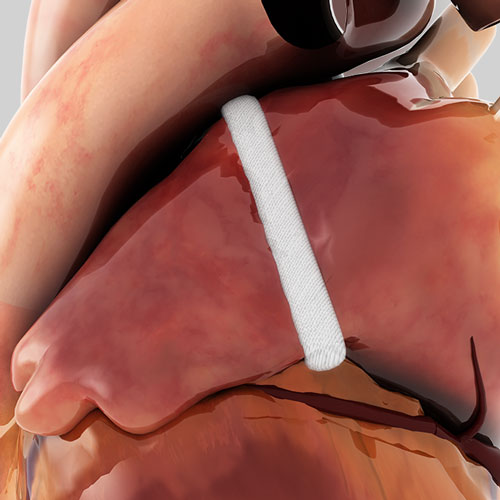
The AtriClip family of devices are the only Left Atrial Appendage Management (LAAM) devices clinically proven to provide both mechanical1 and electrical isolation of the LAA.2
LAAM is now supported with Class IA recommendations from ACC/AHA and STS. This is based on decades of experience and clinical data supporting the efficacy and safety of the AtriClip System.
AtriCure’s AtriClip devices are the most widely implanted LAAM devices in the world, providing a permanent solution to LAA exclusion during cardiac procedures.
AtriClip Devices Sold
Years of Expertise and Innovation in LAAM
Published Studies Including AtriClip Devices
MR CONDITIONAL
The LAA Exclusion System Clip is MR Conditional. A patient with the LAA Exclusion System Clip may be safely scanned under the following conditions. Failure to follow these conditions may result in injury to the patient: