Product Features
- 12 mm port compatibility
- Open-ended design clip
- Tip-first closure

Tip-first closure to capture the entire base of the LAA

Titanium Alloy Construction
Patients with a nickel hypersensitivity may benefit from left atrial appendage (LAA) closure with an AtriCure V-Clip LAA exclusion device, which is constructed with titanium alloy.*
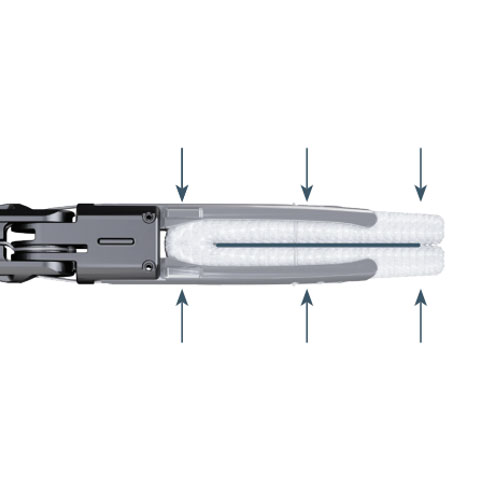
Continuous Dynamic Closing Pressure
The AtriClip Device applies continuous dynamic closing pressure to the base of the LAA as it atrophies.
AtriClip Device Performance with an Open-Ended Design
See how the AtriClip PRO•V Device is used in procedures
- PROV35 (35 mm)
- PROV40 (40 mm)
- PROV45 (45 mm)
- PROV50 (50 mm)
INDICATIONS:
The AtriClip LAA Exclusion System is indicated for the exclusion of the heart’s left atrial appendage, performed under direct visualization and in conjunction with other cardiac surgical procedures.
MRI SAFETY INFORMATION:
MR CONDITIONAL
The LAA Exclusion System Clip is MR Conditional. A patient with the LAA Exclusion System Clip may be safely scanned under the following conditions. Failure to follow these conditions may result in injury to the patient:
- Nominal Values of Static Magnetic Field: 1.5-Tesla or 3.0-Tesla
- Maximum Spatial Field Gradient: 40 T/m (4,000 gauss/cm)
- Type of RF Excitation: Circularly Polarized (CP) (i.e., Quadrature Transmission)
- Transmit RF Coil Information: There are no transmit RF coil restrictions.
- Operating Mode of MR System: Normal Operating Mode
- Maximum Whole Body Averaged SAR: 2-W/kg (Normal Operating Mode)
- Limits on Scan Duration: Whole body averaged SAR of 2-W/kg for 60 minutes of continuous RF exposure (i.e., per pulse sequence or back-to-back sequences/series without breaks)
- MR Image Artifact: The presence of this implant produces an imaging artifact. Therefore, carefully select pulse sequence parameters if the implant is located in the area of interest.
*The AtriCure V-Clip LAA exclusion device may contain trace amounts of nickel, typically less than 0.05%.