The FDA approved the EPi-Sense System to treat patients diagnosed with long-standing persistent atrial fibrillation (LSPAF). This is the first and only FDA approval of a minimally invasive ablation therapy with the EPi-Sense device for more than 4 million patients in the United States with LSPAF.1 This approval significantly expands atrial fibrillation treatment options for long-standing persistent patients.
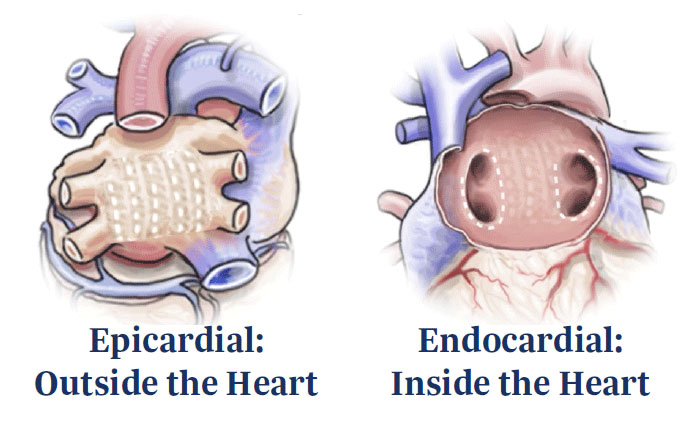
- Minimally invasive pericardioscopic epicardial ablation
- Endocardial radiofrequency (RF) catheter ablation
Epicardial ablation uses RF energy applied to the posterior left atrial wall, distal from the esophagus. The aim is to create durable and contiguous lesions while reducing risk of injury to the adjacent structures of the heart.
Endocardial RF ablation employs mapping and ablation to target the regions requiring additional treatment, as well as those areas impeded by pericardial reflections during the epicardial procedure. Pulmonary vein isolation (PVI) completes the lesion set.
Clinical Data Support Efficacy for Long-Standing Persistent Patients
The prospective, superiority, randomized, controlled CONVERGE IDE trial revealed the success of Hybrid AF Therapy compared to endocardial RF catheter ablation alone.2,3 Highlights include the following.
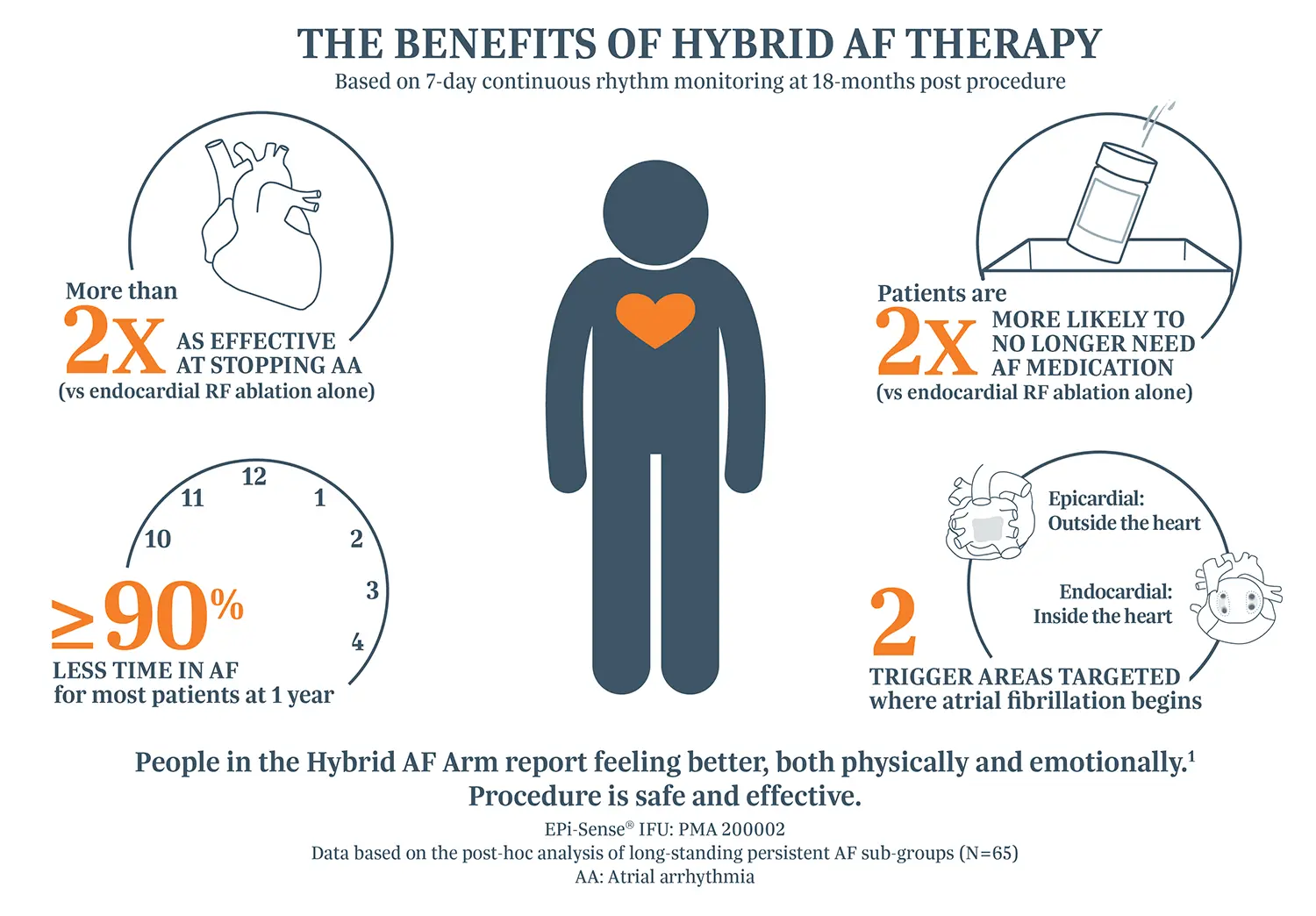
Quality of Life
People in the Hybrid AF Arm report feeling better, both physically and emotionally. Procedure is safe and effective.3
Catheter Ablation Alone for LSPAF
Catheter ablation is often effective for paroxysmal atrial fibrillation. However, a recent Consensus Statement points out that the efficacy of endocardial catheter ablation to treat persistent atrial fibrillation and LSPAF is 30-40%.4
Prevalence of Atrial Fibrillation
One in 4 adults over 40 will develop atrial fibrillation in their lifetime.5 And approximately 45% of AF patients have LSPAF6—which affects more than 4 million patients in the United States,1 who until now had very few effective treatment options available.
PM-US-1673C-1026-G
Products
| Learn more about Hybrid AF Therapy Products |
|---|
Links
- Clinical Data
- Resources
- Press Release: Hybrid AF Therapy for the Treatment of Long-Standing Persistent Atrial Fibrillation
EPi-Sense Coagulation System/EPi-Sense ST Coagulation Device
The EPi-Sense Coagulation System/EPi-Sense ST Coagulation Device is intended for the treatment of symptomatic long-standing persistent atrial fibrillation (continuous atrial fibrillation greater than 12 months duration) when augmented in a hybrid procedure with an endocardial catheter listed in the instructions for use, in patients (1) who are refractory or intolerant to at least one Class I and/or III antiarrhythmic drug (AAD); and (2) in whom the expected benefit from rhythm control outweighs the potential known risks associated with a hybrid procedure such as delayed post-procedure inflammatory pericardial effusions. Contraindications include patients with Barrett’s Esophagitis, left atrial thrombus, a systemic infection, active endocarditis, or a localized infection at the surgical site at the time of surgery. Adverse Events: Reported adverse events associated with epicardial ablation procedure may include, but are not limited to, the following: pericardial effusion/cardiac tamponade, pericarditis, excessive bleeding, phrenic nerve injury, stroke/TIA/neurologic complication. Warnings: Physicians should consider post-operative anti-inflammatory medication to decrease the potential for post-operative pericarditis. and/or delayed post-procedure inflammatory pericardial effusions. Physicians should consider post-procedural imaging (i.e. 1-3 weeks post-procedure) for detection of post-procedure inflammatory pericardial effusions. Precautions: Precautionary measures should be taken prior to considering treatment of patients: (1) Deemed to be high risk and who may not tolerate a potential delayed post-procedure inflammatory pericardial effusion. (2) Who may not be compliant with needed follow-ups to identify potential safety risks. To ensure patients undergoing treatment with the EPi-Sense/EPi-Sense ST device are well informed, the benefits, potential risks and procedural outcomes associated with the EPi-Sense/EPi-Sense ST Hybrid Convergent procedure should be discussed with the patient. Physicians should document accordingly in the medical record. Qualified operators are physicians authorized by their institution to perform surgical sub-xyphoid pericardial access. The coagulation devices should be used by physicians trained in the techniques of minimally invasive endoscopic surgical procedures and in the specific approach to be used. Operators should undergo training on the use of EPi-Sense/EPi-Sense ST device before performing the procedure. Safety and effectiveness of concomitant left atrial appendage closure was not evaluated in the CONVERGE study. Follow-up should be conducted at approximately 30 days postprocedure to monitor for signs of delayed onset pericarditis or pericardial effusion.
References:
- Noubiap, J, Tang, J, Teraoka, J. et al. Minimum National Prevalence of Diagnosed Atrial Fibrillation Inferred From California Acute Care Facilities. JACC. 2024 Oct, 84 (16) 1501–1508. Persistent patient estimate: Berisso et al Epidemiology of atrial fibrillation: European perspective Clin Epidemiol. 2014; 6: 213–220
- DeLurgio, D.B., et al. (2021). Hybrid epicardial-endocardial RF ablation vs. endocardial catheter ablation for long-standing persistent atrial fibrillation treatment: Results from CONVERGE randomized controlled trial. International AF Symposium.
- IFU for EPi-Sense® Guided Coagulation System Data: PMA# P200002.
- Calkins H, et al. (2017). 2017 HRS/EHRA/ECAS/APHRS/SOLAECE Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm, 14(10).
- Lloyd-Jones, D.M., et al. (2004). Lifetime risk for development of atrial fibrillation. Circulation, 110, 1042-1046. doi: 10.1161/01.CIR.0000140263.20897.42.
- Colilla, S. et al. (2013). Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. American Journal of Cardiology, 112(8), 1142-1147.

