As a leading provider of innovative technologies for the treatment of Atrial Fibrillation (Afib) and related conditions, electrophysiologists, cardiothoracic and thoracic surgeons around the globe count on AtriCure to deliver best-in-class solutions that can treat even the most complex cases. Our Isolator® Synergy™ Ablation System is the first medical device to receive FDA approval for the treatment of persistent Afib. Our AtriClip® Left Atrial Appendage Exclusion System products are the most widely sold LAA management devices worldwide. Our Hybrid AF™ Therapy is a minimally invasive procedure that provides a lasting solution for long-standing persistent Afib patients. Our cryoICE cryoSPHERE®+ and cryoSPHERE MAX™ probes are cleared for temporary ablation of peripheral nerves to block pain, providing pain relief in cardiac and thoracic procedures. We invest in innovation, clinical science, and education to focus on improving lives for our patients worldwide.
Mission
We are passionately focused on healing the lives of those affected by Afib and pain after surgery.
Values
We work to heal the lives of Patients, empower our People, and collaborate with our Partners to reduce the burden of Afib worldwide.
Patients
Our first responsibility is to put patients first. We lead rigorous clinical science to determine the safest, most effective approaches and treatments. Our commitment to education generates consistent, predictable practices and standards of care. We undertake relentless innovation to reveal new ideas that improve the experience of providers and supports the care they deliver to each patient.
People
Our people act with unwavering integrity and transparency in all we do. While honoring the dignity of each person, we foster collaboration to achieve excellence across all areas. We sustain a humble culture of gratitude for each other and the good that we can bring to the world.
Partners
We are committed to delivering the highest quality and most efficient solutions to benefit our partners, including care providers, customers, and shareholders. We strive to understand their needs and to offer the products, services, and business value that meet those needs.
Key Facts
- Served over 650,000 patients with ablation worldwide
- Over 4,600 healthcare professionals trained
- Products sold in approximately 50 countries
- Over 750,000 AtriClip devices sold worldwide
- 220 issued patents (USA)
- More than 1,300 employees worldwide
- Over 450 scientists and engineers
- Headquartered near Cincinnati, Ohio
- Developed first and only device approved by FDA for surgical treatment of persistent and long-standing persistent Afib
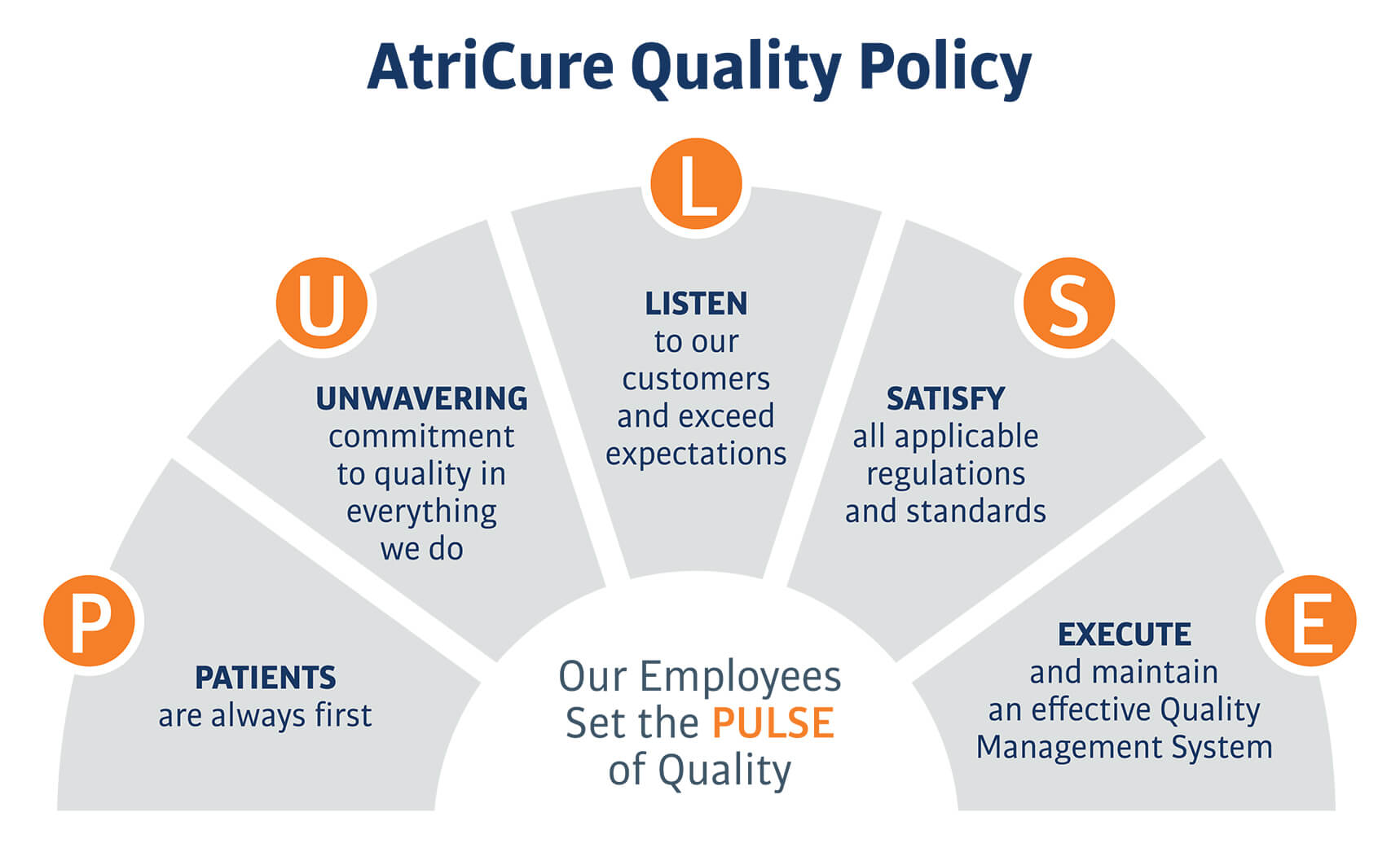
Our Quality Policy
AtriCure’s quality policy represents its commitment to complying with quality standards and regulations.
Awards and Recognition


